Tips for set-up to prevent complications in robotic gynecologic cancer surgery
Article information
Abstract
Robotic surgery (RS) is a breakthrough in gynecologic oncology in the past decade and it is now part of the routine in many centers. Although there is a concern about the oncological outcomes after RS in cervical cancer, it is proven to be safe and effective in endometrial cancer. However, RS has some drawbacks such as the loss of tactile feedback. Complications can therefore occur, and are usually multi-factorial. These can be related to the nature of the operations, the physical fitness of the patients, the control and choice of the devices, and more importantly, the experience and knowledge of the surgical team. To minimise the risk of complications, we need to understand the limitations of RS and have a proper set-up of the operations. It is important to anticipate these potential complications before and during the operations. And a careful setup of the operations, including the instrument set-up, patients’ positioning, port placement, and communication with the anesthetists and surgical team, are crucial in ensuring the safety and success of robotic surgery. In this article, different complications and their preventive measures at set-up were discussed.
INTRODUCTION
The first robotic system, ‘Arthrobot’, was introduced in 1983 [1]. After that, many different models had been developed. In 2000, the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) was approved by the Food and Drug Administration, United States, and it was subsequently approved for gynecologic surgery in 2005. Since then, robotic surgery (RS) has been widely adopted in different types of benign and oncological operations like sacrocolpopexy, radical hysterectomy, pelvic and para-aortic lymphadenectomy. Although data from the Laparoscopic Approach to Cervical Cancer trial showed a worsened overall survival after minimally invasive surgery in cervical cancer [2], robust data demonstrated the safety of RS in other cancers including endometrial cancer. In a recent systematic review that studied 30 articles, RS was associated with less estimated blood loss, shorter length of hospitalization (LOH), lower incidence of intraoperative complications and rate of readmission compared to conventional laparoscopic surgery and laparotomy [3]. The three-dimensional view, magnification, dexterity and seven-degree motion allow access to deep and difficult areas and improve the precision of surgery. Nevertheless, complications can occur in about 10% of RS [4], and this is partly due to inadequate preparation of the surgery. Therefore, a proper set-up, both pre-operatively and intra-operatively, can greatly facilitate the smooth running of the operations and ensure the safety of the patients. In this article, the potential problems and troubleshooting during the set-up of RS were discussed.
ANTICIPATED COMPLICATIONS
The first step to prepare the operations is to anticipate the potential complications. The causes of complications are usually multi-factorial, which can arise from the inherent risks of the operations and RS, or factors related to the patients, the devices and the surgical team (Fig. 1). These factors may interact with each other, which then directly or indirectly influence the patients’ positioning, pneumoperitoneum, and length of operation intraoperatively, thus leading to complications. It is the ultimate responsibility of the whole surgical team to minimize the risks of complications.
1. Inherent risks of operations
Gynecologic cancer operations frequently require deep pelvic dissection which is in proximity of bladder, ureters and bowel. RS has a lack of tactile sensation and limited surgical field of view, and these may account partly for the occurrence of organ injury. Meta-analyses showed that the risk of bladder and ureteric injury after robotic staging surgery was similar to open and laparoscopic surgery [5,6] and the overall incidence was less than 3% [7,8]. Such urological complications can occur by sharp injury, thermal injury or devascularization during the dissection of ureters and bladder, especially at the vesicovaginal ligaments during radical hysterectomy where there are many tiny vessels [9]. The incidence of bowel injury in RS was 1% or less [7,8,10]. The most common injured site was colon and rectum (37.5% each), followed by small bowel (24%) [10]. Bowel injury could occur during primary port entry, grasping by traumatic forceps, sharp or thermal injury during adhesiolysis, hysterectomy or para-aortic lymphadenectomy [10,11].
Common robotic gynecologic cancer surgery like pelvic and para-aortic lymphadenectomy can be associated with vascular injury. The incidence was about 1% [7,8], and was a major cause of conversion to laparotomy [12]. This can be caused by poor surgical view where the vessels are beyond the field of view, inadvertent clashing of robotic instrument which the surgeons are not aware of, or activating the wrong foot energy pedal.
Subcutaneous emphysema (SCE) was rarely reported in gynecologic surgery [13-16]. Extrapolating the experience from laparoscopic surgery, risk factors for SCE include multiple abdominal entries, long operative times of ≥200 minutes, five or more laparoscopic ports, and age older than 65 years [17,18]. A retrospective of robotic prostatectomy showed that BMI <25 kg/m2 and partial pressure of end-tidal CO2 of ≥ 46 mmHg were two independent factors for SCE [19]. SCE can lead to laryngeal edema, tracheal compression, and subsequently hypercarbia and respiratory acidosis [17]. The treatment of hypercarbia by increasing pulmonary ventilation, however, is limited by Trendelenburg position and pneumoperitoneum [19].
2. Risks related to RS
Patients undergoing RS especially upper abdominal surgery require a steep Trendelenburg position at 20-30 degree (Fig. 2). Such position, together with pneumoperitoneum, could induce laryngeal edema, restrict the movement of the diaphragm and chest wall, reduce the lung compliance, and increase plateau airway pressure and end-tidal CO2 pressure [20,21]. The overall complication rate is 0.8% [22], and intolerance to Trendelenburg position led to 6% of robotic hysterectomy conversions [23]. This unphysiological position and pneumoperitoneum could also affect the autonomic nervous system, slower the heart rate, increase the mean arterial pressure, and increase the cardiac shunt fraction [24,25]. Fortunately, there was no effect on the cerebral oxygenation [26,27].
In addition, prolonged steep Trendelenburg position may rarely cause head contusion, raised intracranial pressure (ICP) and optic nerve sheath diameter, subcutaneous ecchymosis, peri-orbital edema and pain, corneal abrasion, and even visual loss [22,28-31]. Nonetheless, in a cohort including 28 robotic hysterectomies, the intra-ocular pressure, retinal nerve fiber layer thickness and other parameters in most patients did not have a significant change from pre-operative parameters 3 months after the operation [32].
To prevent falling from the Trendelenburg position, the patients have to be protected by braces, pads and mattresses. However, over pressure of these may induce palsy of the brachial plexus, common peroneal nerve, femoral nerve, obturator nerve and sciatic nerve [28,33-35]. Prolonged lithotomy may also cause compartment syndrome and rhabdomyolysis up to 0.3% [35-38].
3. Patients’ factors
Certain complications can be attributed by the patients’ age, physical fitness, past surgical history, body habitus and type of procedures. For example, patients with history of pelvic abscess, deep infiltrating endometriosis, multiple laparotomies or radiotherapy are at a higher risk of organ injury than normal population due to possible adhesion and distorted anatomy. In endometrial cancer, a certain proportion of patients are obese and old. Previous articles demonstrated the safety of RS in obese and morbidly obese (BMI ≥40 kg/m2 ) patients [23,39]. RS was also associated with less blood loss, shorter LOH and lower conversion rate than those undergoing laparoscopic surgery in endometrial cancer patients [40-44], though the benefit of shortening the operative time was controversial [43,44]. RS also did not have adverse impact on the disease-free survival and overall survival compared to open surgery in endometrial cancer patients [45,46]. Similarly, RS was safe and feasible in elderly in endometrial cancer too [47-49]. A recent meta-analysis analyzed five studies showed that there was a reduction of overall and peri-operative complications (relative risk [RR] 0.40 and 0.43, respectively), as well as shorter LOH by 3.3 days, compared to laparotomy [50].
Nevertheless, it is not uncommon for obese and elderly patients to have other comorbidities like pre-existing cardio-vascular diseases. It is unclear whether steep Trendelenburg position and pneumoperitoneum have any adverse effects on these patients [51-53]. On the other hand, without such position and pneumoperitoneum, the bowel loops may prolapse to the lower abdomen making the operation difficult, especially when para-aortic lymphadenectomy or other upper abdominal procedures is needed.
4. Machinery factors
Machinery faults may occur during the set-up. However, these errors usually have self-explanatory solutions by the pop-up messages or the LED indicators on the robotic arms. In fact, the arms of new robotic systems are slimmer and there is auto-target function, making the docking and alignment of arms easier and faster.
Insulation failure of robotic instrument used to be more prevalent than laparoscopic instrument (32% vs. 13%) [54], which could lead to vascular or organ injury. This problem was less reported nowadays, possibly because of a better design of the instrument and/or the overall improvement in the surgical techniques and instrument handling over time. However, improper handling of instrument can still occur as described above.
5. Surgeons’ factors
About 20% of complications are operator-dependent [55]. Some could be due to unfamiliarity with the patients’ condition and anatomy, and some could be due to poor visualization of surgical field, clashing of robotic instrument or forceful grasping of tissues. All these might directly or indirectly prolong the length of operation. Catanzarite et al. [56] showed that operative time of ≥240 minutes could result in more complications including venous thromboembolism, blood transfusion, and re-operation in benign laparoscopic and robotic hysterectomy. And each additional hour of operation time was estimated to increase the above risk by 1.2 to 1.5 times.
PREVENTIVE MEASURES AT SET-UP
Many of the above complications may be prevented with a proper set-up of the robotic operations.
Before the operations, the surgeons need to assess the patients’ fitness and review the indications and modes of the operations which may be adjusted accordingly. For example, patients with severe cardiopulmonary disease may not be fit for pneumoperitoneum and steep Trendelenburg position, and laparotomy may have to be considered. Similarly, patients with history of pelvic inflammatory disease or severe endometriosis may be more prone to organ damage. The role of prophylactic ureteric stenting in preventing ureteric injury is unclear [57-59], and laparotomy may be a better option. All the treatment options, including the procedures, risks and alternatives, should be explained to the patients thoroughly and the discussion should be documented properly. For patients who have significant medical comorbidities, it is necessary to communicate with different medical teams to optimize their conditions, and discuss with the anesthetists on the use of anesthesia. For example, patients with hypertension, stroke or brain tumor may better receive propofol instead of sevoflurane anesthesia as the former caused less increase in ICP and optic nerve sheath diameter [60]. Special attention should be paid to patients with extreme body mass index (BMI) or big uterus and is discussed as below.
1. Patients with low BMI
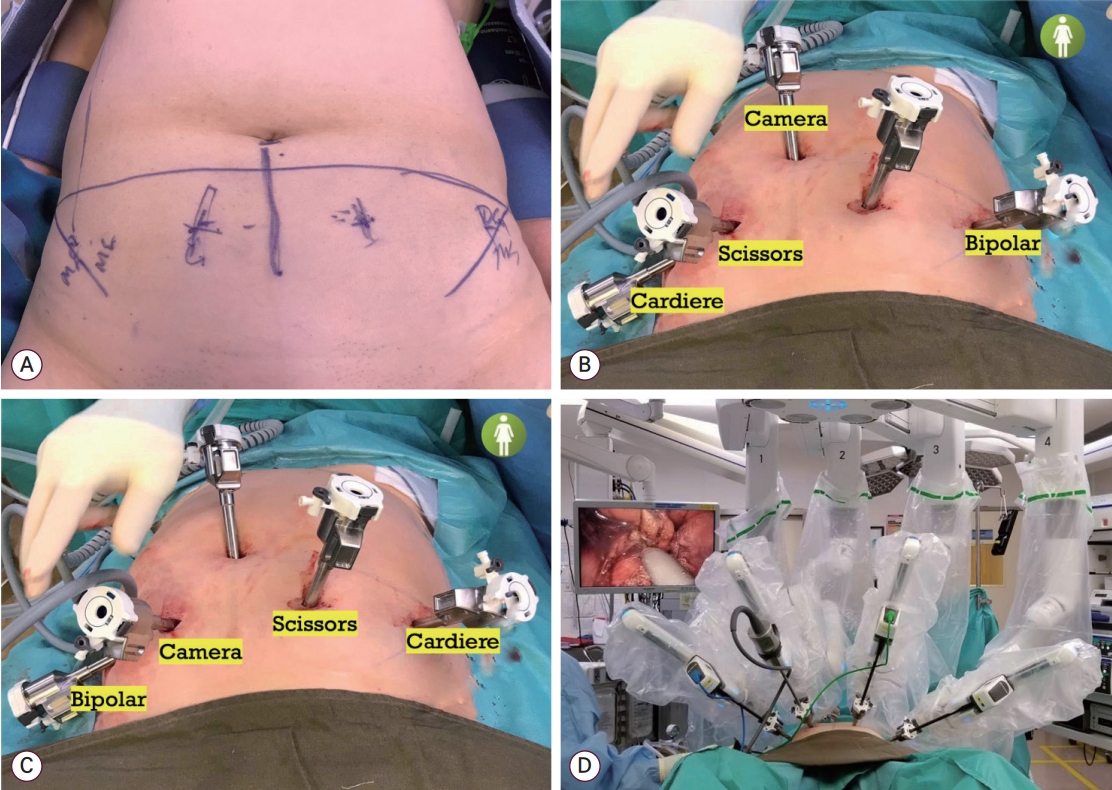
It is not uncommon for Asian patients to have low BMI. First, for surgeons who do not use Hasson’s open entry technique, care must be taken when inserting Veress needle during the primary port entry to avoid vascular injury, and the needle should be inserted at a direction of 45 degree or less in thin patients [11]. The surface area of the abdomen in this group is usually small, and there may not be enough room to accommodate so many ports. The surface area of the upper abdomen is further reduced for those who have narrow costal angle and long rib cage. If the ports are squeezed in upper abdomen, there is a risk of clashing of instrument, which in turn may restrict the movement of the robotic arms and increase the risk of organ damage. To tackle this problem, the surgeons can consider using the lower pelvic port placement method [61]. The robot, da Vinci Xi (Intuitive Surgical Inc.), is docked from the right leg of the patient. The primary port is placed at the umbilicus. Four more ports are placed at 1-2 cm above the level of the anterior superior iliac spines, equally apart spanning from either side of the mid-axillary lines (Fig. 3). An assistant port is placed at the right upper quadrant. When operating at the pelvis, the camera is placed at the umbilicus, the bipolar is placed at the left lateral port, the monopolar is placed at the right medial port and the grasper is placed at the right lateral port. When operating at the upper abdomen, the boom is turned 180 degree clockwise. The camera is placed at the right medial port of the patient. The other instruments are placed reciprocally as how they are placed for pelvic surgery. For some patients who have short body built, the right medial port is placed 1-2 cm below the other pelvic ports so that the camera can have a broader field of view when operating at the upper abdomen.
2. Patients with high BMI
For morbidly obese patients, it is important to protect the patients by enough protective pads and mattress to avoid slipping down during Trendelenburg position and extensive flexion, extension and abduction of the joints and limbs. The anesthetists may consider using pressure-controlled ventilation (PCV) volume-guaranteed or PCV, instead of volume-controlled ventilation, to increase the mean inspiratory pressure, tidal volume and dynamic lung compliance [62]. The patients should be kept euvolemic. Some advocated to restrict fluid replacement to less than 1,500-2,000 mL to reduce the risks of laryngeal, pharyngeal and facial edema [63-65]. Long trocars and instrument should be used to allow room for the instrument to move and reach the target organs. Extra assistant ports and fan-shaped retractor may be needed to help retracting the bowel. Valveless system (Airseal, ConMed, Largo, FL, USA) can be considered as this can maintain a stable pneumoperitoneum at a relatively low intra-abdominal pressure, typically at 12 mmHg [66,67]. This system also provides constant smoke evacuation and allows easy passage of gauze, specimens, and needles, which may potentially reduce the risk of SCE. For patients who need both hysterectomy, pelvic and para-aortic lymphadenectomy, one can consider operating at the upper abdomen first before operating at the pelvis. This modified sequence of steps can allow steeper Trendelenburg and more muscle relaxants at the beginning which can be reduced when operating at the pelvis subsequently. For those who are vulnerable and may not be able to tolerate the whole RS, hysterectomy may have to be performed first before the staging procedure. If expertise is available, extraperitoneal lymphadenectomy can be considered for those who cannot tolerate Trendelenburg position or pneumoperitoneum.
There were recommendations on the peri-operative care in obese and elderly patients [65,68,69]. Smith et al. [69] demonstrated that the implementation of a high BMI pathway could shorten the operating time, lower the estimated blood loss, and result in fewer complications compared to the period without such pathway. Fornalik et al. [65] also showed that using a dedicated protocol, robotic lymphadenectomy was safe and had a higher yield of lymph nodes compared with open lymphadenectomy in obese patients. A summary of some important approaches is listed in Table 1.
3. Patients with big uterus
For patients who have big uterus, the gas can be insufflated by inserting a Veress needle at the Palmer’s point after inserting a nasogastric tube [70]. The camera port can be put above the umbilicus for pelvic operations, and the right medial port can be placed further down when lower pelvic port placement is used. Non-traumatic uterine elevator like McCartney’s tube (Symmetry Surgical Inc., Antioch, TN, USA) can elevate and manipulate the direction of the uterus to avoid close contact and thermal injury to the nearby organs. A retrieval bag like Endobag (Medtronic, Minneapolis, MN, USA) can be used to retrieve the uterus so that it can be bisected or morcellated in the close bag in the vagina. If the uterus cannot fit in the retrieval bag, mini-laparotomy can be considered at the end of the operation.
CREDENTIALING PROGRAMES
Certainly, the ultimate success of any RS lies with the surgeons’ knowledge on anatomy and peri-operative care, the familiarity with the instrument, the procedures of the operations, and the communication with the nurses, assistants and anesthetists. A well-structured training programme and credentialing system can provide a guidance for the surgeons to acquire the skills step by step and ensure the surgeons’ capability to perform RS safely [71,72]. The Asian Society for Gynecologic Robotic Surgery has also made a recommendation on the credentialing programme [72]. Briefly, the surgeons are required to have enough experience on open procedures and knowledge on the basic principles of RS. Then the surgeons need to perform at least five procedures under supervision or proctoring to obtain the credentialing. Subsequently, the surgeons have to maintain the skills with certain caseload or through simulation training afterwards. Periodic audits may also be required at the institution levels to improve the quality of care.
CONCLUSION
RS allows a clear visualization of the anatomy and access to difficult areas, and provide a favourable ergonomic environment for the surgeons. However, there are limitations with the system. A structured training programme and having a proper set-up with an experienced team are crucial for the safe and efficient execution of RS. For vulnerable groups like those who are morbidly obese or old, a dedicated management pathway can provide a platform for different specialties to follow and facilitate the smooth running of the operation and ensure a safe peri-operative course.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.